Moderna Booster Half Dose
1 day agoThe US. Cutting the Moderna doses in half could help.
In addition to Moderna potentially adding a half-dose booster Pfizer is seeking authorization of a vaccine for children ages 5 to 11 with a 10-microgram dose -- one-third the strength given to.

Moderna booster half dose. FDA Weighing Dose of Moderna Covid-19 Booster Vaccine maker has asked agency to authorize booster at half the dose given in the first two shots. 2 days agoThe US. The Food and Drug Administration is leaning toward approving a half-dose of Modernas COVID-19 vaccine as a booster shot according to a.
Food and Drug Administration is leaning toward authorizing half-dose booster shots of the Moderna Inc. That would mark the second COVID-19 vaccine to get authorization from the FDA for booster doses. Federal health officials may authorize a half-dose of Modernas COVID-19 vaccine as a protective booster shot.
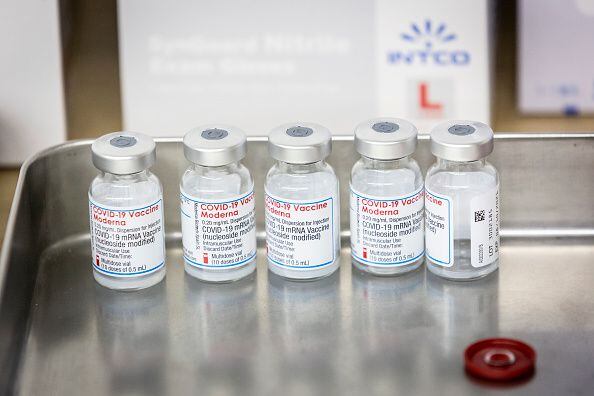
Moderna meanwhile is focused on halving its booster shot from the current 100 microgram dose. The US Food and Drug Administration is leaning toward authorizing half-dose booster shots of the Moderna Inc COVID-19 vaccine Bloomberg News reported on Tuesday citing. 15 hours agoModerna submitted data to the FDA seeking evaluation for its booster shot on Sept.
The drugmakers original vaccine contains 100 micrograms of mRNA in each shot. 2 days agoThe US Food and Drug Administration is leaning toward authorizing half-dose booster shots of the Moderna Inc Covid-19 vaccine Bloomberg News reported citing people familiar with the matter. We are pleased to initiate the submission process for our booster candidate at the 50 µg dose with the FDA.
Food and Drug Administration is on the verge of authorizing half-dose booster shots of the Moderna COVID-19 vaccine according to Bloomberg News. 30 2021 -- The FDA is leaning toward authorizing half-dose booster shots of Modernas COVID-19 vaccine. If proved effective the reduction in Modernas booster dosage could significantly expand the global vaccine supply.
7 hours agoThe half-dose decision could reduce the side effects of the shot according to BloombergAnd it would allow Moderna to distribute more doses throughout the world over the long term. Modernas booster study reported that adults aged 65 and above saw the greatest improvement in protection and that the half dose appears to be effective against all variants of concern. The agency is satisfied that the dosage level would provide enough protection against the virus people familiar with the matter told Bloomberg News.
3 hours agoThe Food and Drug Administration is leaning toward approving a half-dose of Modernas COVID-19 vaccine as a booster shot according to a report. Coronavirus vaccine satisfied that its effective in shoring up protection people. Fauci has indicated that therell be progress soon on booster shots for Moderna as well as Johnson Johnsons one-dose vaccine.
2 days agoSEPTEMBER 29 2021 0538. 6 hours agoModerna says new data supports need for booster shot. 2 days agoIn addition to Moderna potentially adding a half-dose booster Pfizer is seeking authorization of a vaccine for children ages 5 to 11 with a 10-microgram dose -- one-third the strength given to.
People who are older than 65 years old or who have underlying conditions can get the booster shot now. The Food and Drug Administration is reported to be leaning toward approving a lower-dose version of Modernas covid shot as a booster. Food and Drug Administration FDA is leaning toward authorizing half-dose booster shots of the Moderna Inc COVID-19 vaccine Bloomberg News reported on Tuesday citing people familiar.
Why would the Moderna booster shot be a half dose. Moderna had pushed for. 1 day agoModernas initial inoculations contained 100-microgram doses and the companys submission to regulators amounted to a push to authorize a.
The agency had been looking at the. 20 hours agoHalf-Dose Moderna Vaccine May Be Recommended By FDA As Booster. Plans to authorize a Moderna COVID-19 booster vaccine are ramping up and now the Food and Drug Administration is reportedly leaning toward a half dose as a booster shot Bloomberg reportedModernas push for a follow-up COVID-19 shot for the fully vaccinated comes as booster shots are increasingly a hot topic of discussion across medical and scientific.
The findings have not yet been peer-reviewed. The company submitted to the FDA an. Bloomberg citing people familiar with the matter reported this week that the US.
Federal regulators believe the. The Pfizer vaccine has already been authorized by the FDA for booster shots. 19 hours agoModerna had pushed for the half-dose shot in its booster application submitted to federal regulators on Sept.
1 day agoIn addition to Moderna potentially adding a half-dose booster Pfizer is seeking authorization of a vaccine for children ages 5 to 11 with a 10-microgram dose -- one-third the strength given to those 12 and up. Modernas current vaccine shot is a 100-microgram dose compared with Pfizers 30-microgram dose. Food and Drug Administration was leaning toward authorizing half-dose booster shots for those who received Modernas two-shot mRNA vaccine.
A 50-microgram dose appears to be effective enough to increase waning vaccine.
Fda Leans Toward Authorizing Moderna Booster At A Half Dose
/cdn.vox-cdn.com/uploads/chorus_image/image/69935052/Mass_Vax_Site_Presser_sg_09.0.jpg)
Moderna Booster Shot May Only Be Available In Half Doses Here S Why Deseret News

U S Fda Leaning Toward Approving Moderna Half Dose Booster Report Winnipeg Sun

Boosters For Moderna And J J Recipients Not Up For Debate At C D C Panel The New York Times

Fda Considering Half Dose Of Moderna Vaccine As Booster Fox Business


:quality(70)/d1hfln2sfez66z.cloudfront.net/09-29-2021/t_4f725635448141f3aeb1f78f3993cc85_name_FDA_may_soon_authorize_Moderna_booster_s_615487d73cae215649f869da_1_Sep_29_2021_15_59_54_poster.jpg)






/cloudfront-us-east-2.images.arcpublishing.com/reuters/FI4A6YULCVJ6HFVAHGGUB4RBZI.jpg)
Posting Komentar untuk "Moderna Booster Half Dose"